Glory Info About How To Tell If Its A Redox Reaction

It also looks at how you go about choosing a suitable.
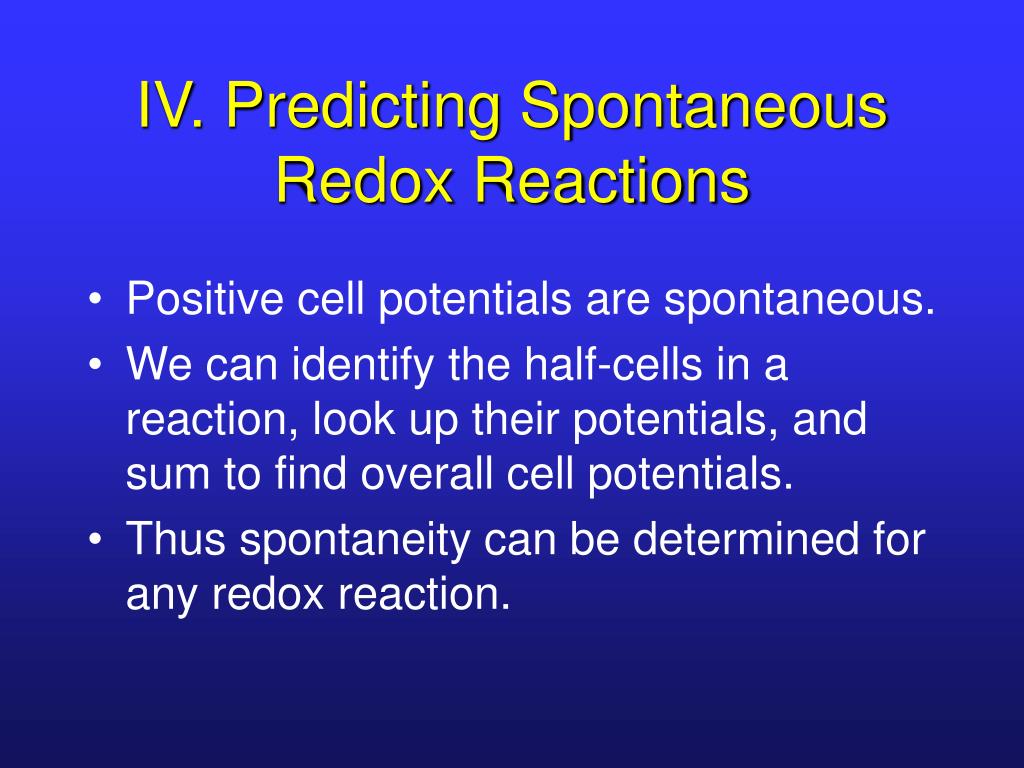
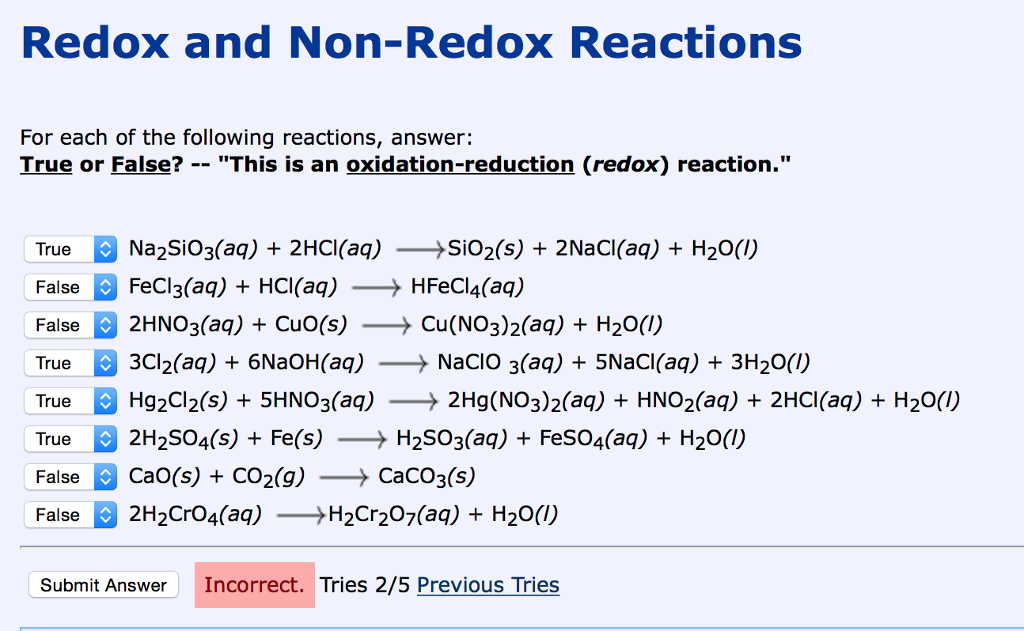
How to tell if its a redox reaction. Equations for redox reactions can be produced by adding together the two ion. This page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. We use the following rules to assign oxidation numbers.
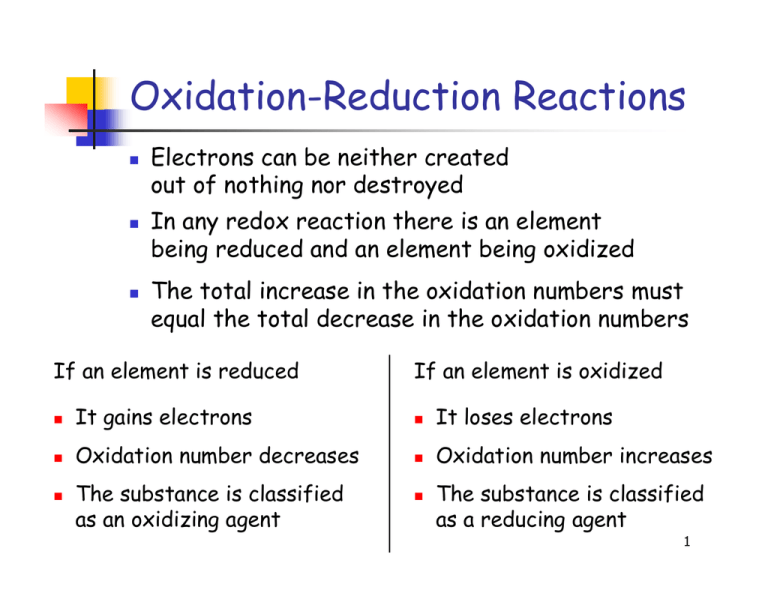
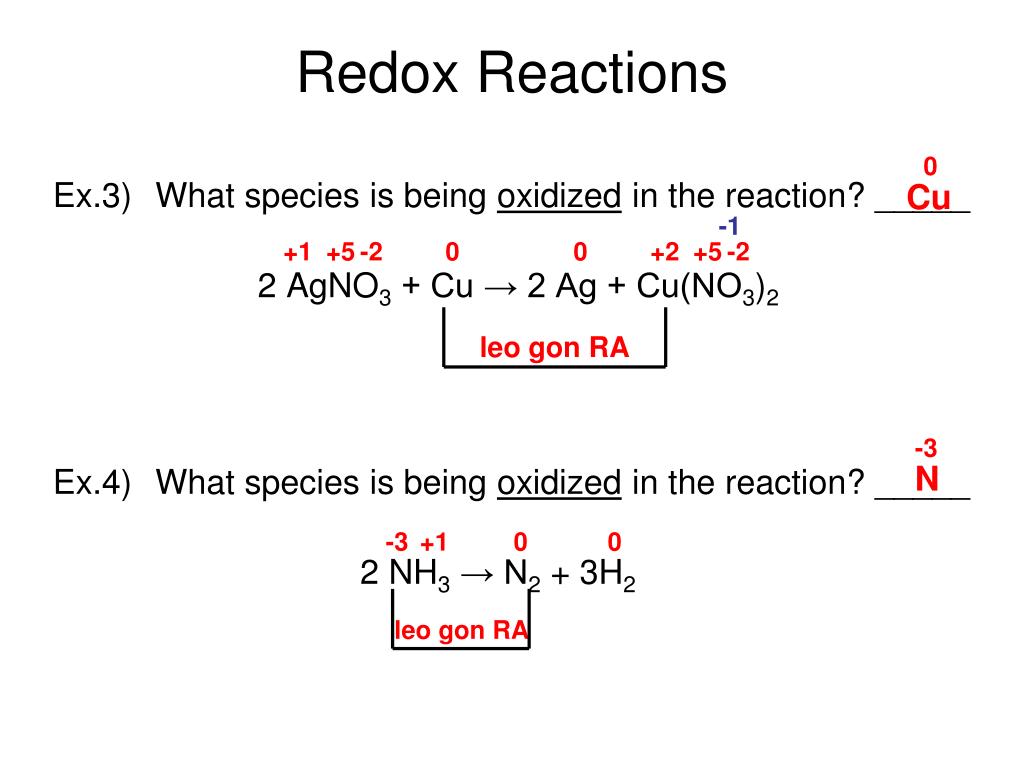
Redox reactions are reactions in which electrons shift allegiance. For each of the reactions given below, calculate the oxidation number of each of the elements in the reactants and the products and determine if the reaction involves. Identifying oxidation and reduction identify which species is oxidized and which is reduced by looking at the changes in oxidation numbers.
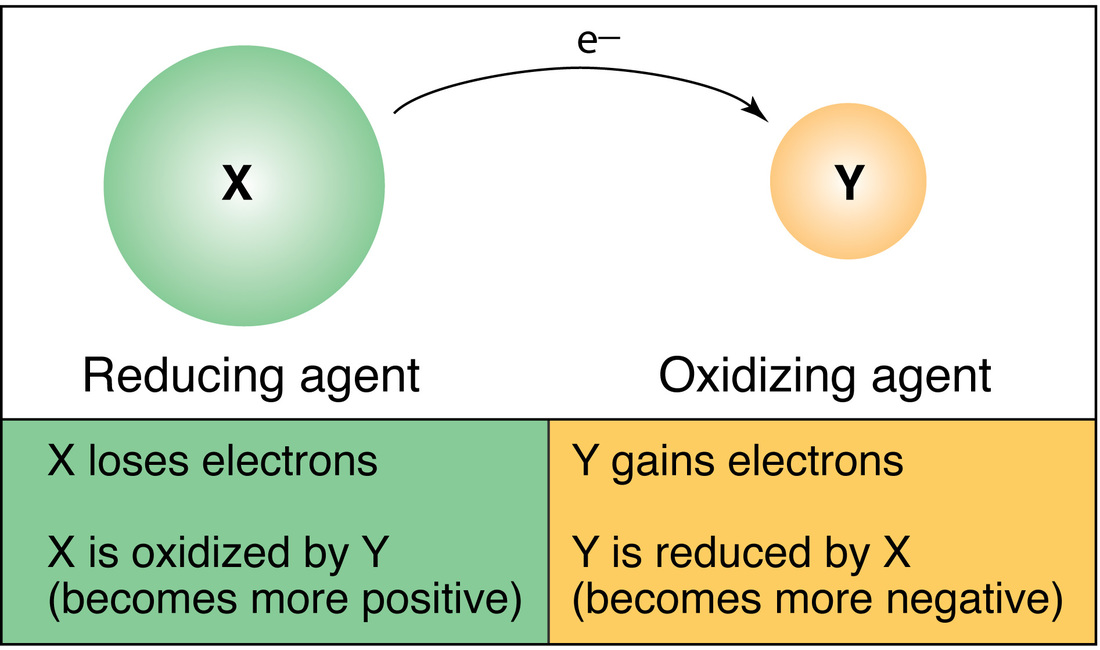
A redox equation can be balanced using the following stepwise procedure: Oxidation is the loss of electrons.
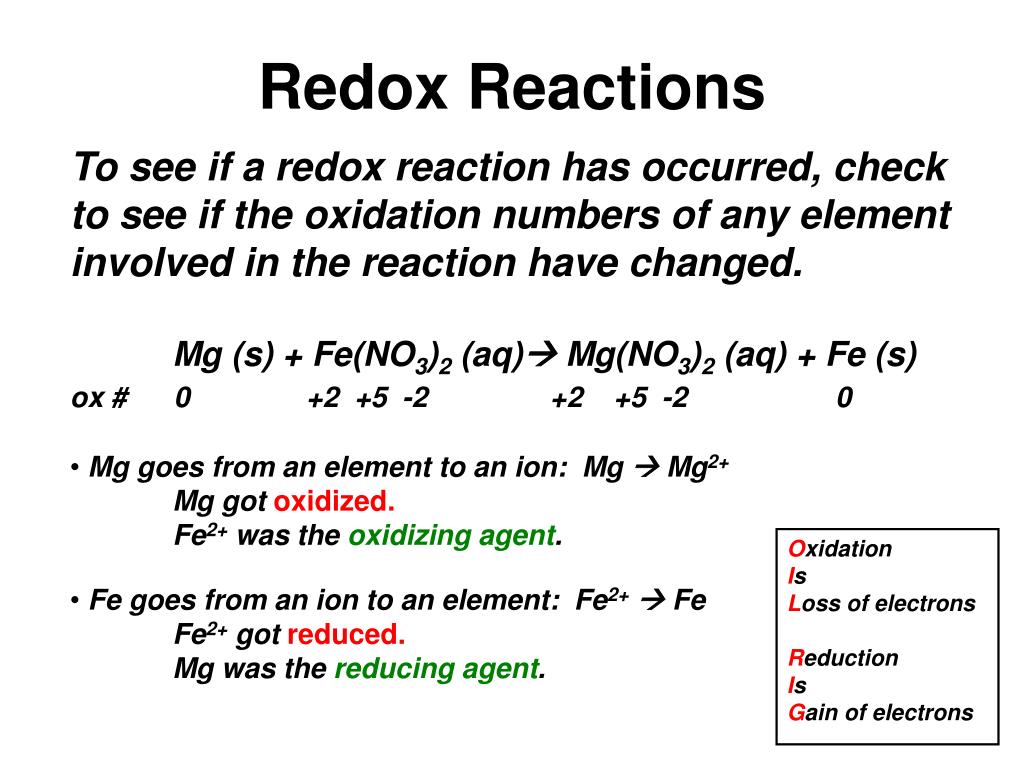
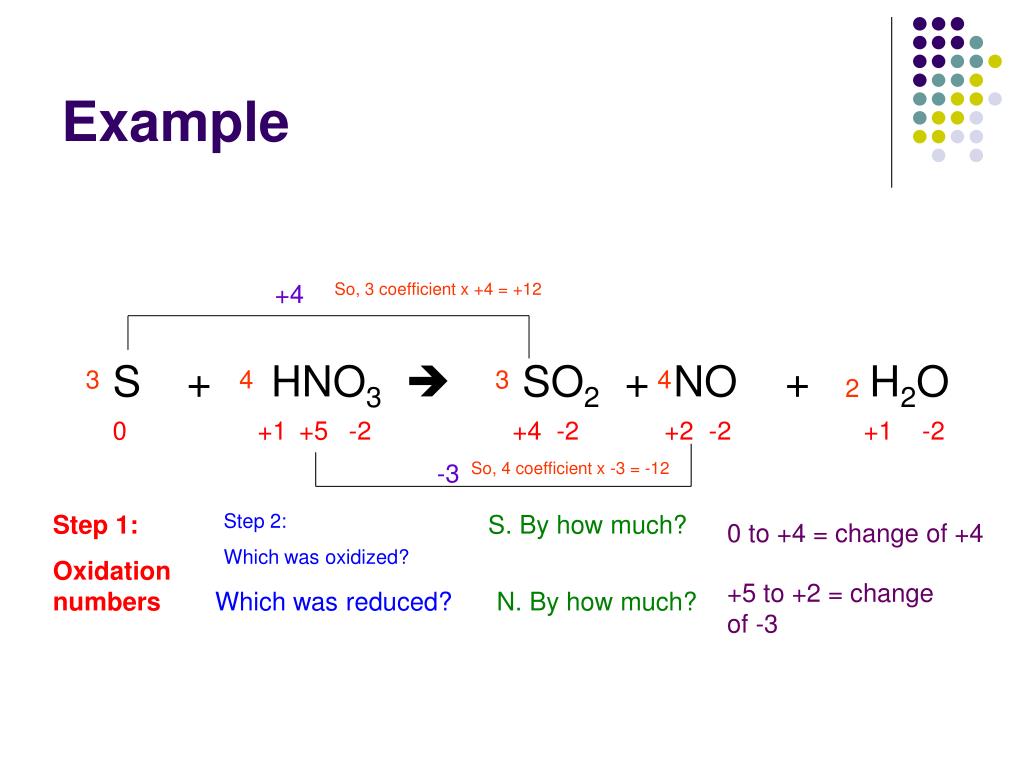
To identify a redox reaction, we must first calculate the oxidation number of each atom in the reaction. This video explains how to identify. To identify a redox reaction, first, we need to know the oxidation status of each element in the reaction.
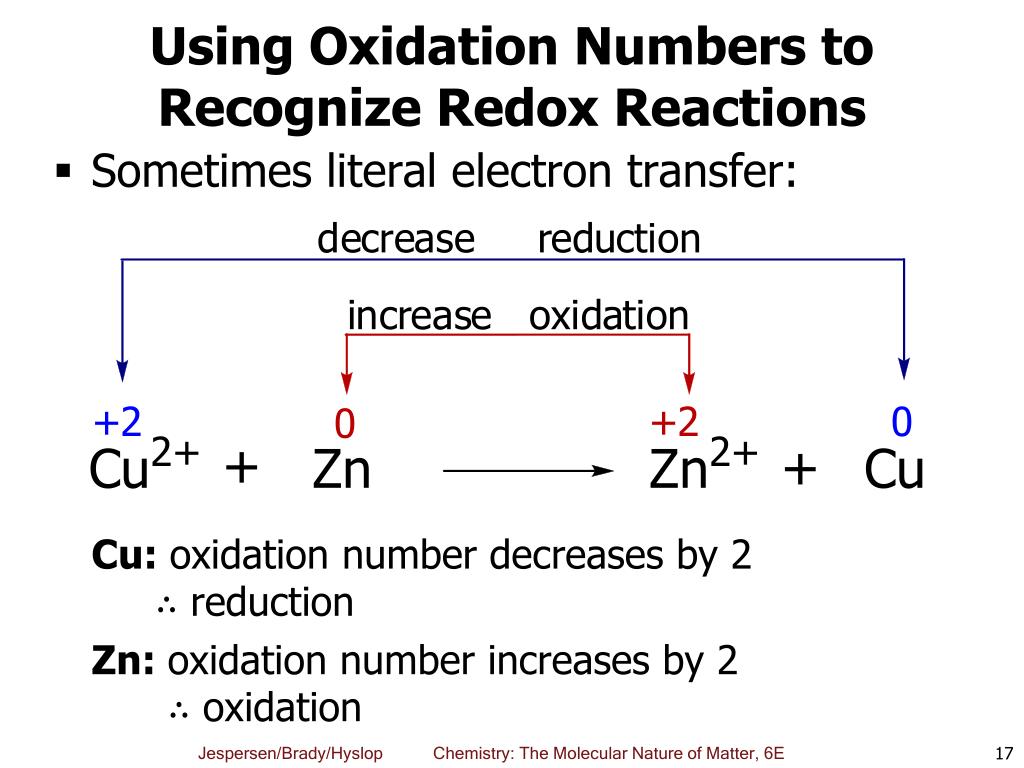
By assigning oxidation numbers to the atoms of each element in a redox equation, we can determine which element is oxidized and which element is reduced during the reaction. Allegiance means loyalty or commitment to a group, like your allegiance to. Use oxidation number to determine which species in a reaction is oxidized and which is reduced, which is the oxidizing agent and reducing agent.
How to identify a redox reaction? A redox reaction is one in which both oxidation and reduction take place. At least two elements must change their oxidation numbers.
This chemistry video tutorial provides a basic introduction into oxidation reduction reactions also known as redox reactions. If there is no change in oxidation number, then the reaction is not a. All redox reactions occur with a simultaneous change in the oxidation numbers of some atoms.
Ask question asked 7 years, 10 months ago modified 5 years, 7 months ago viewed 48k times 6 which balanced equation. Any reaction in which no oxidation.